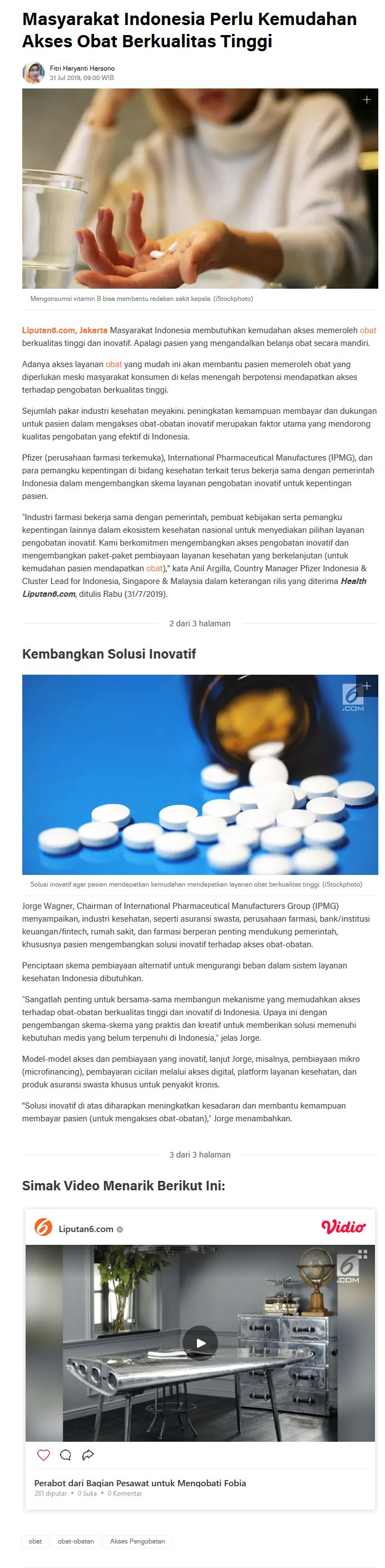
VISION & MISSION
IPMG seeks to be the trusted partner to the government of Indonesia and other stakeholders and to play an important role in improving the healthcare system through the core strengths of its members; i.e., medical innovation and manufacturing of products that meet international standards of pharmaceutical quality and safety.
COMMITMENT
As an organization, IPMG is committed to working with all healthcare sector stakeholders and to fully support government programs and regulations. IPMG and its members assiduously strive to ensure Indonesians have access to quality healthcare products.
Read MoreROLE
IPMG communicates the commitment and contribution of R&D-based pharmaceutical manufacturers in broadening access to innovative medicines, vaccines and biologics as well as in advocating and providing recommendations for the advancement of an effective and integrated healthcare system in Indonesia
Read More